Basic Operation
600 MHz NMR
“In the absence of a magnetic field the period of all these oscillations is the same. But as soon as the electron is exposed to the effect of a magnetic field, its motion changes.” – Zeeman, P.
FIRST PROCEDURES
Content:
1. Sample preparation
2. Some important line commands
3. Data acquisition
3.1. Locking
3.2. Shim
4. Annexes
5. References
1. Sample preparation
“There are no good results using bad samples.”
CAUTION! Clean the sample of precipitates, cells debris, bubbles, foam, any solid and heterogeneous phases at all in your sample, because different phases will cause distortion on the field and will impact the result. To deal with this issue, use ultracentrifuge in a highest speed as possible to deposit any solid, and/or filter the sample using at least 0,22 µm pore size filter (attention to the filter composition to avoid dissolution of the material by the solvent) right before fill the NMR tube. This step is crucial to obtain a well-adjusted shimming applied to your experiment.
ALAWAYS! Clean and dry the sample tube. A tiny scratch, humidity, oily, or dust can destroy the high quality properties of the tube making it useless for high resolution experiments, and also leaving dirty to the inside of the probe, reducing the sensitivity and harming the magnet.
Make your sample using a deuterated solvent (often deuterated water is used for polar samples at 10-20% v/v) until the total volume reaches a volume of ~ 500 to 600 µL (or 0.5 – 0.6mL) in a 5-mm tube, 3.1 mL in a 10-mm tube, and 150 µL (or 0,15 mL) for a Shigemi® tube are adequate for removing the end effects. In general, the NMR samples are compatible with: Acetone-d6, chloroform-d, methylene chloride-d2, and DMSO-d6. Make sure that the solvent will not react or degrade the sample and has a high purity for NMR, free of any impurity. Remember, the peaks of the impurity will appear in the same spectrum of your sample, which can cause a misinterpretation of the result and loss of the sensibility (gain).
If the impurity is part of the solvent, is possible to dry the contaminated solvent through N2 stream and dissolving the sample in an appropriate solvent again (repeat the process if you observe any improvement on the result, usually monitored by 1D NMR screening is enough.
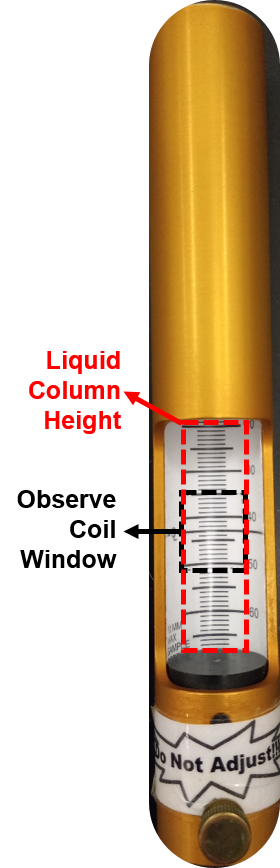
The liquid column length must be at least three times the length of the observe coil window to minimize end effects (fig. 1). A typical sample length is 5 cm regardless of the diameter of the NMR tube used, positioned properly. Make every sample up to the same height in order to obtain similar shim values using samples of that height.
ATTENTION! Some probes may require particular volume and height adjustments. Reduction of sample volume to attain higher concentration usually fails, at this case some special plugs for low volume samples are available and will help with line shape.
Figure 1. Sample depth gauge:
2. Some important line commands
“No command, no work.”
3. Data acquisition
“Experts often possess more data than judgment.” - Powel, C.
Always remember because these experiments involve a fourier transform that to double your signal-tonoise
ratio requires four times as many scans. So if you can’t see anything after 1h you are not going to have
much to look at after 4h, likewise if you can not see anything after 2h it is not going to improve a lot after 8h.
So on long experiments check your data after an hour or so and if can not see any correlations you might as well
stop the experiment.
3.1. Locking
3.2. Shim
5. References:
NMR Spectroscopy, User Guide Varian, Inc. Inova and MercuryPlus NMR Systems With VnmrJ 2.2MI Software Pub. No. 01-999378-00, Rev. A 0708